- Home
- Products
- Water Measurements
- Analytical Technology Inc.
- Permanganate
- Q46H/83 Permanganate Monitor
Q46H/83 Permanganate Monitor
- General Details
- Features
- Documents
Continuous water quality monitoring of permanganate in treated water presents sensor contamination issues. When permanganate reacts with organics or other reducing materials in solution, manganese dioxide (MnO2) is formed and readily plates out on sensing electrode surfaces. The resulting deposits degrade the measurement and can be difficult to clean. The Q46/83 eliminates this problem by employing a measurement method in which the sensor never comes into contact with the sample. In operation, water containing permanganate is mixed with pH buffer and potassium iodide solutions. Permanganate oxidizes the iodide to iodine (I2), and the resulting I2 is stripped out of solution and measured using an I2 gas sensor. This “gas phase” measurement technique eliminates MnO2 sensor fouling, resulting in a system capable of providing long term reliability.
The Q46H/83 Permanganate water quality monitor consists of two main components, an electronic display unit and a chemistry module. The chemistry module contains sample and reagent pumps, sample handling systems, and the gas sensor that provides the final measurement. An inlet overflow assembly attached to the bottom of the chemistry module is where the inlet sample line and drain line are connected. Sample inlet flows of 250-100 ml./min. are recommended to keep system response time to a minimum. Reagent bottle holders are supplied so that reagents can be wall mounted below the chemistry system.
The electronics module can be mounted near the chemistry unit or up to 100 feet away depending on installation requirements. Permanganate concentration is displayed on a large format back-lit LCD with a second alpha-numeric line below to indicate other operating parameters. Three SPDT alarm relays and two 4-20 mA outputs are provided, and digital communications are available.
A Gas-Phase Approach to Sulfide Measurement
THEORY OF OPERATION
The Q46H/83 Permanganate Monitor employs ATI’s Auto-Chem chemistry module to provide chemical treatment of the sample, air stripping of the released iodine, gas sample conditioning, and iodine gas measurement. In operation, 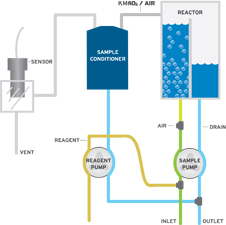 a small amount of sample is pumped from an overflow assembly into the chemistry system and mixed with pH 4 buffer and potassium iodide (KI) solution. At pH 4, permanganate compounds in solution react as follows:
a small amount of sample is pumped from an overflow assembly into the chemistry system and mixed with pH 4 buffer and potassium iodide (KI) solution. At pH 4, permanganate compounds in solution react as follows:
2MnO4- + 6I-
3I2 + 2MnO2
The treated sample flows into an air-stripping chamber where a controlled amount of air removes the iodine from the water sample. The air coming from the top of the stripping chamber is directed to a gas conditioning module which removes any excess water and then to a flowcell containing the iodine gas sensor. The iodine gas sensor is an amperometric membraned sensor that generates current in proportion to the amount of iodine in the gas stream. Since the gas sensor is only in contact with clean air containing iodine, sensor fouling due to contaminants in the sample is eliminated.
Gas Phase Sensing
Measurement is made without contact between sample and sensor, eliminating the potential for sensor fouling.
Standard Method
Potassium Permanganate is measured using idometric measurement after reaction of the sample with buffer and potassium iodide.
Analog Output Options
Two isolated 4-20mA outputs are standard. One output is programmable for PID function.
Chemistry Module Power Options
Power options include 115 or 230 VAC, 50/60 Hz.
Three Control Relays
Relays are programmable for setpoint, deadband, and time delay.
Digital Communications
Communication options for Profibus-DP, Modbus-RTU, or Ethernet-IP.
Clear Display
Back-lit large LCD display provides clear visibility in any lighting condition. A scrolling second line on the display provides additional information and programming prompts.


